Why Abzena?
Our focused approach.

We are committed to offering a fully integrated US and UK network. This brings together mAb production, conjugation, sterile filling, lyophilization, and packaging – helping de-risk, secure and stay ahead of shifting geopolitical and supply-chain dynamics that could knock your critical programs off-track.
Learn more about how Abzena can simplify and secure your supply chain here.
At Abzena we offer a focused approach with:
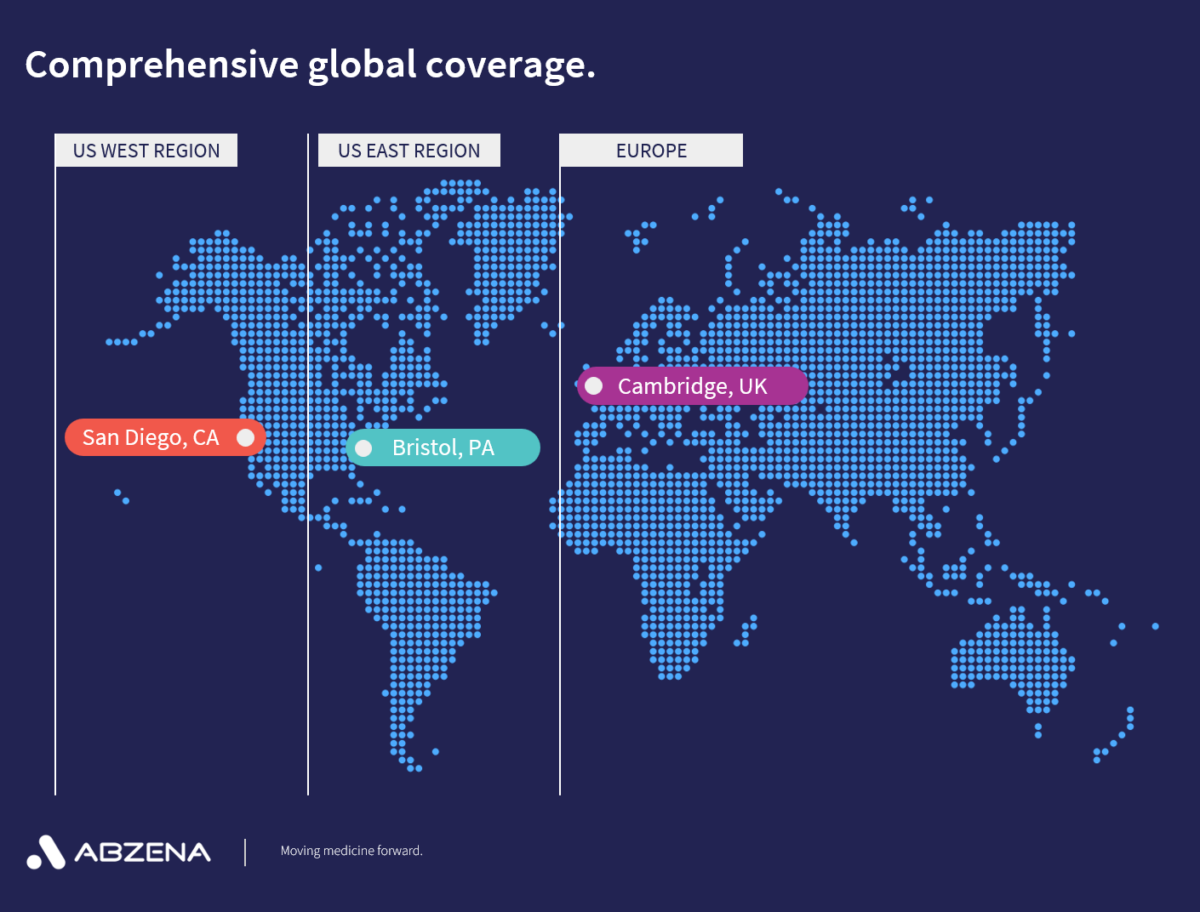
Abzena has research, development, and cGMP facilities across locations in San Diego, CA, Bristol, PA, and Cambridge, UK, Our services and solutions have been tailored to move your biologic and bioconjugate development programs forward at every stage in the process.
Click below to access our corporate brochure to learn why we are considered to be the leading bioconjugate, antibody-drug conjugate (ADC), and complex biologics CDMO + CRO.
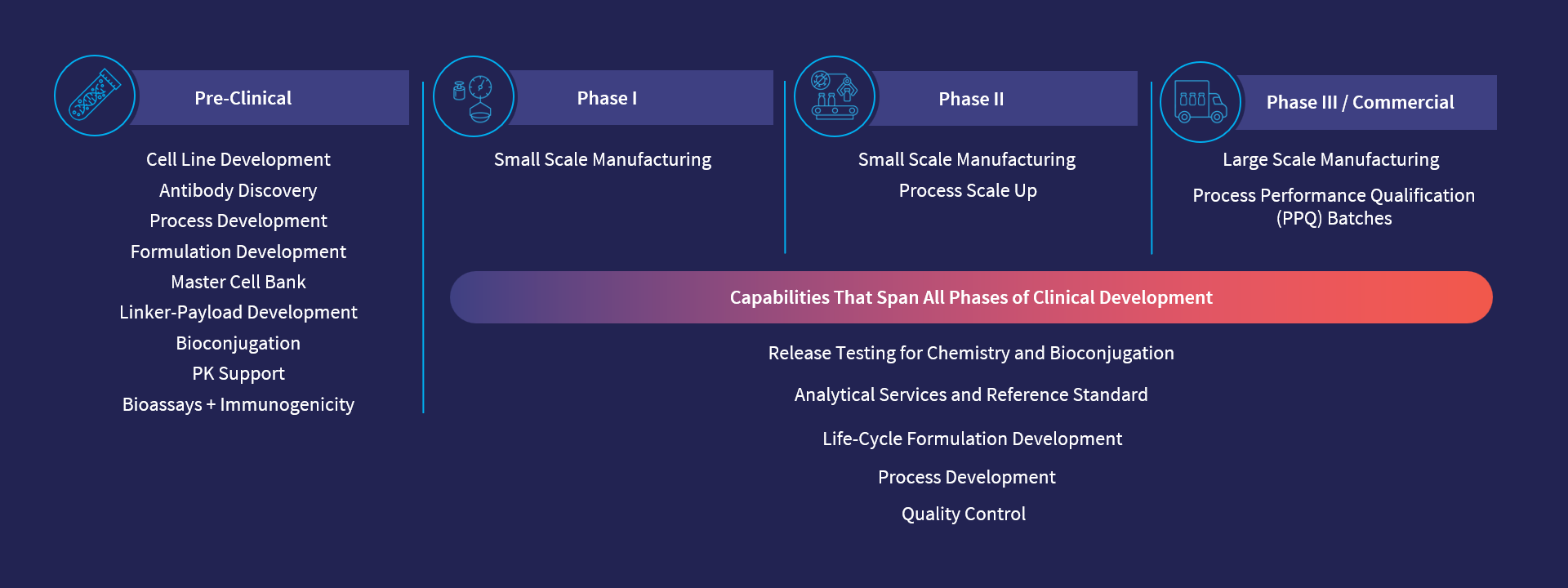
From early discovery through commercial, our experienced scientists work with you side-by-side, functioning seamlessly as part of your team — using real-world insights to bring new ideas to the table and then turning them into action. With quality at the front of everything we do, we plan the best route, steering your drug program toward regulatory approval, and getting it quickly and effectively to patients.
We have over 20 years of experience in delivering solutions at each stage of the development lifecycle from early-stage research and discovery, through lead candidate selection, and onward into process development and GMP manufacture.
Unlike most other service providers, we can provide technical and scientific support that truly spans early-stage research through process development and GMP manufacturing under a single organization to better ensure that the TPP can be achieved. Our global scientific teams have extensive experience in overcoming some of the most challenging and complex biologic and bioconjugate programs, making us the perfect partner for novel and disruptive technologies that other CDMOs may shy away from.
Our experienced team is focused on getting it right from the start. We identify and address challenges early in drug design to better ensure downstream clinical and commercial success. We also have expertise in fixing and optimizing drugs in later stage development to rescue them from the drug development graveyard.
With capabilities ranging from bioassays to cell line development, bioconjugation and clinical and commercial manufacturing, all under one organization, we can reduce the white space in the development path, which ultimately minimizes risk, reduces costs and accelerates timelines for our customers.
As an integrated service provider, we accelerate timelines through the ease of technical and materials transfer, and improved scheduling. Better workflows and processes can also be attained through improved knowledge transfer between internal multidisciplinary experts and the development of a deep understanding of the drug as it progresses from target to lead selection and process development and manufacture.
Our team is always seeking new ways to innovate and streamline development for our customers. Our extensive scientific capabilities and proprietary solutions like EpiScreen® 2.0, Composite Proteins™, Composite Human Antibodies™, AbZelectPRO™, ThioBridge™, and LabZient™ are designed to give your program the best chance of clinical and commercial success.
Lead candidates discovered, and designed.
Customers request additional work.
Integrated programs delivered in support of INDs.
4x programs scaling to PPQ and commercial production. 100+ production cell lines developed.
At Abzena, our unique structure and our values help us build collaborative and long-lasting relationships with our partners. We believe partnering with other organizations allows scientific innovation to flourish. We are a trusted partner across all phases of drug discovery and development, focused on growing together. Our ultimate goal is to get next-generation medicines to patients, faster.