Why Abzena?
Our focused approach.
ThioBridge™ is important for the advancement of ADCs. ThioBridge™ provides several benefits for ADC development as it addresses issues commonly associated with traditional bioconjugation technologies by:
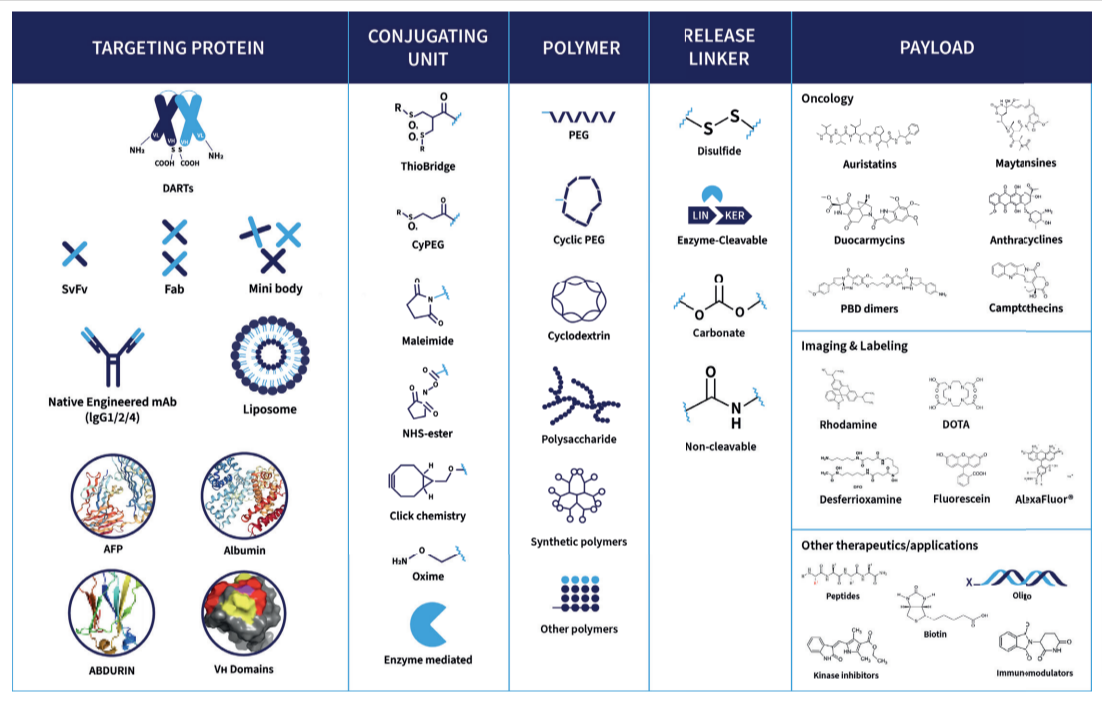
Developed by our scientific experts, Abzena’s ThioBridge™ Antibody Drug Conjugation (ADC) platform is a solution that leverages 20+ years of our experience and data to address some of the challenges and learn from early successes that we have seen in ADC development. Fundamentally, ThioBridge™ conjugation overcomes many of the issues seen in existing technologies to give our customers ADCs improved stability, potency, and efficacy.
While ADCs are a powerful and targeted approach to therapeutics, first-generation conjugation technologies have limitations such as inconsistent drug-to-antibody ratios (DAR) and the risk of early payload detachment.
Abzena’s ThioBridge™ conjugation technology offers a solution by providing a more uniform DAR profile, stable linker attachment, and improved pharmacokinetic properties.

Download our case study today and learn more about how our ThioBridge™ technology. Discover how ThioBridge™ can improve the potency, efficacy, and stability of your ADC program as it bridges from preclinical into the clinic and beyond.
If you are looking to rapidly move your ADC program to its next regulatory or clinical milestone, contact us today and learn more about our ThioBridge® technology and how we can support you and your ADC development program.