Why Abzena?
Trust our focused approach.

The leading biologics CDMO + CRO that accelerates drug development, moving medicine forward for patients in need.
Our experienced professionals offer the kind of personalized service and flexible working relationship that you need, and your time-sensitive programs deserve.
We are not afraid to question assumptions, and not shy about presenting new and better ideas. Then, we deliver.
What you can come to expect when you partner with Abzena:
Access to Genuine Scientific & Technical Experts
Proactive Solutions for Streamlining Development
Diverse and Interconnected Teams
End-to-End Support Under One Organization
With research, development, and cGMP facilities across locations in San Diego, CA, Bristol, PA, and Cambridge, UK, Abzena is fully prepared with the services and solutions to move your bioconjugate and biologic programs forward at every step in the process.
Working as interconnected teams, our scientific experts help pharmaceutical and biotech companies bring their treatments to market delivering on our mission to move medicine forward.
Click below to access our corporate brochure to learn why we are considered to be the leading bioconjugate, antibody-drug conjugate (ADC), and complex biologics CDMO + CRO.
We have over 20 years of experience in delivering solutions at each stage of the development lifecycle from early-stage research and discovery, through lead candidate selection, and onward into process development and GMP manufacture.
Unlike most other service providers, we can provide technical and scientific support that truly spans early-stage research through process development and GMP manufacturing under a single organization to better ensure that the TPP can be achieved. Our global scientific teams have extensive experience in overcoming some of the most challenging and complex biologic and bioconjugate programs, making us the perfect partner for novel and disruptive technologies that other biopharmaceutical CDMOs may shy away from.
Our experienced team is focused on getting it right from the start. We identify and address challenges early in drug design to better ensure downstream clinical and commercial success. We also have expertise in fixing and optimizing drugs in later stage development to rescue them from the drug development graveyard.
With capabilities ranging from bioassays to cell line development, bioconjugation and clinical and commercial manufacturing, all under one organization, we can reduce the white space in the development path, which ultimately minimizes risk, reduces costs and accelerates timelines for our customers.
As an integrated service provider, we accelerate timelines through the ease of technical and materials transfer, and improved scheduling. Better workflows and processes can also be attained through improved knowledge transfer between internal multidisciplinary experts and the development of a deep understanding of the drug as it progresses from target to lead selection and process development and manufacture.
Our team is always seeking new ways to innovate and streamline development for our customers. Our extensive scientific capabilities and proprietary solutions like EpiScreen® 2.0, Composite Proteins™, Composite Human Antibodies™, AbZelectPRO™, ThioBridge™, and LabZient™ are designed to give your program the best chance of clinical and commercial success.
We’ve built a fully integrated organization to move complex biologics and bioconjugates ahead.
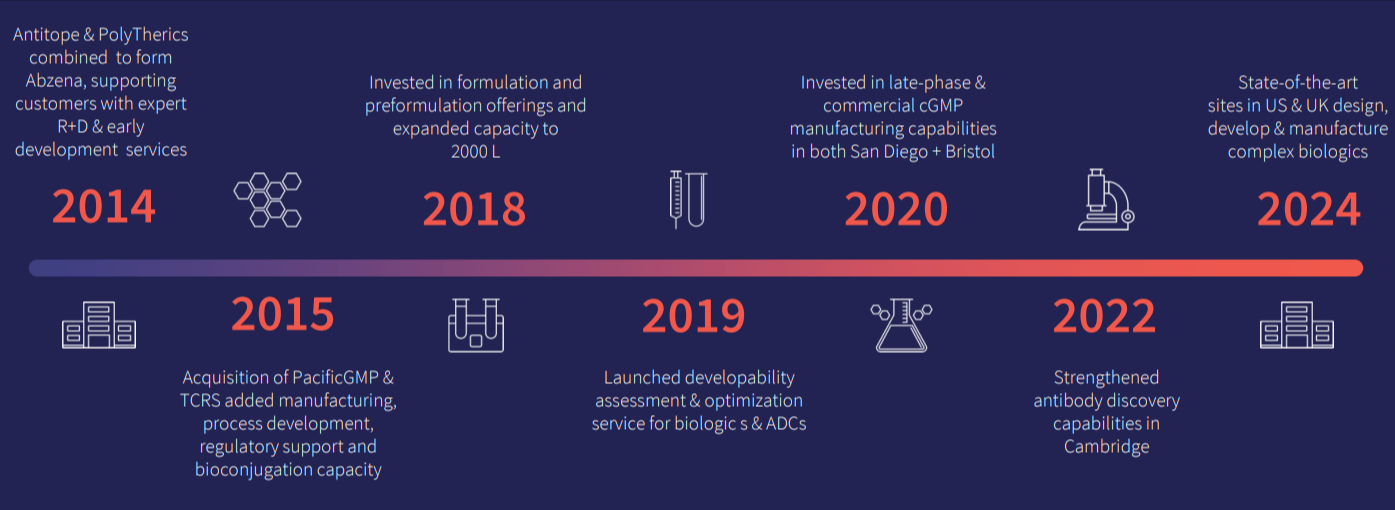
We have over 20 years of experience in delivering solutions at each stage of the development lifecycle from early-stage research and discovery, through lead candidate selection, and onward into process development and GMP manufacture. Learn more about our rich scientific history today!
Abzena for everyone; bring your whole self to work.
You belong. Building an inclusive culture that fosters, empowers and celebrates the diverse voices of our employees, we embrace your thoughts, experiences and talents. It is our belief that the more inclusive we are, the better we work.
We are committed to creating workplaces that reflect the communities we serve so that everyone feels empowered.
Through our values of quality, scientific innovation and integrity, it is our mission to support and uphold fair and just practices and policies in everything that we do.
At Abzena, we place the highest value on our environmental, health, and safety programs. Our Company’s foundation is built on our values which distinguish us and guide our actions.
As Abzena continues to grow and provide essential CDMO services to our partners and their patients, we embrace the opportunity to become an industry leader in corporate sustainability.
Quality is first – to patients, regulatory authorities, and our partners.
Abzena’s Quality organization provides safe and compliant products to your patients. Our Quality organization has a comprehensive program of quality services to ensure compliance with regulatory requirements.
In addition to our expertise, we have the technologies and analytical capabilities to handle any of your modalities; from the simple to the most complex.