Why Abzena?
Trust our focused approach.

Accelerate your timeline to the clinic or the market with our expertise in complex chemistry and bioconjugation services.
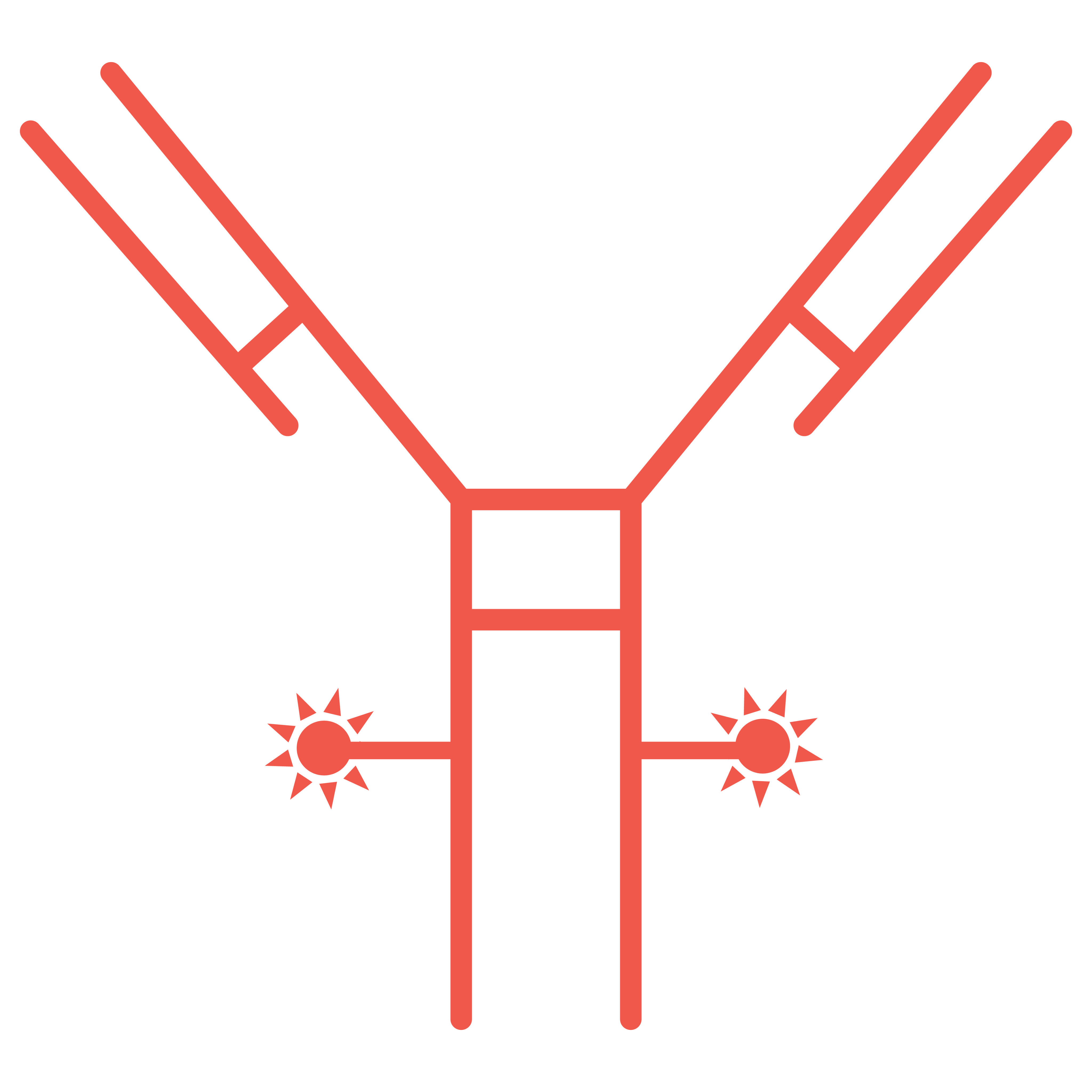
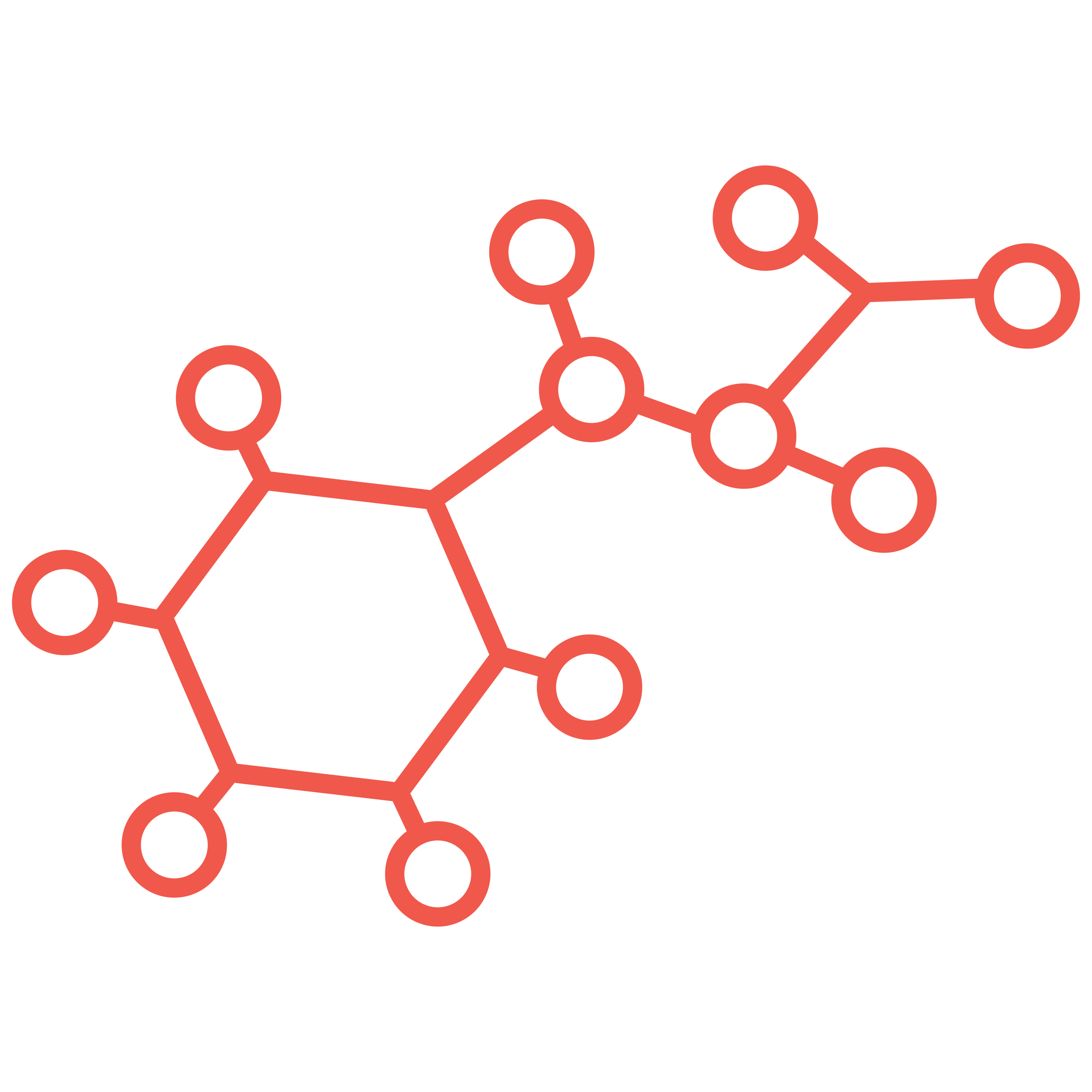
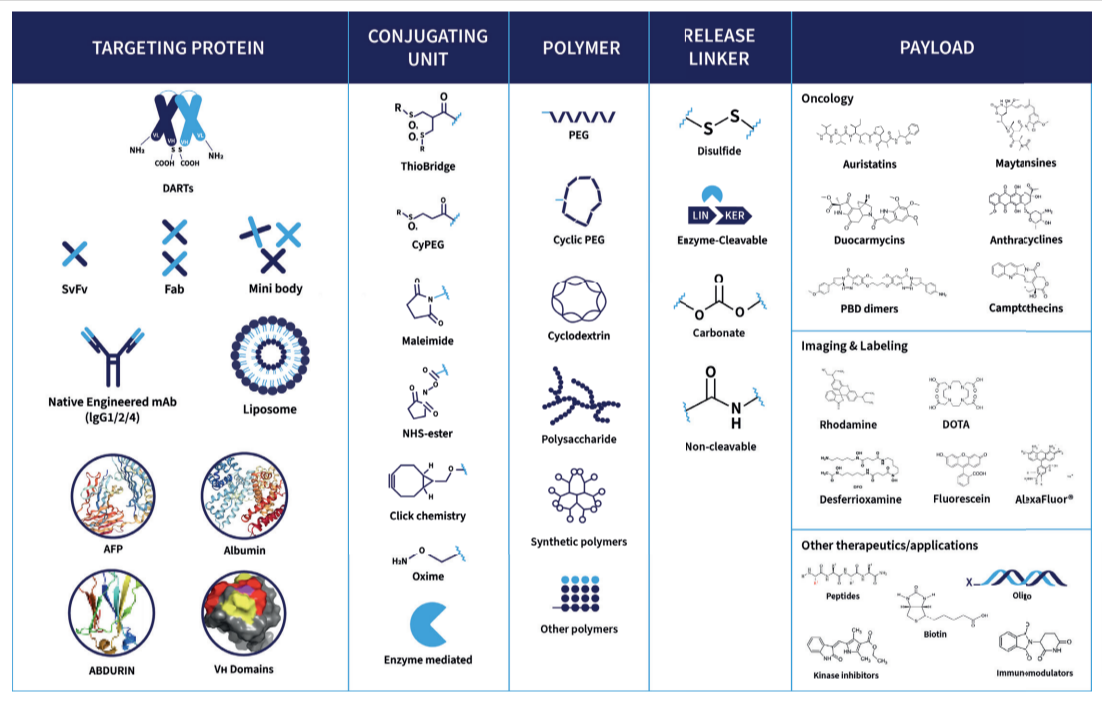
Bioconjugates such as Antibody-Drug Conjugates (ADCs), including Bispecific ADCs, Dual Payload ADCs, Antibody-Oligo Conjugates (AOCs) and Radioconjugates (RDCs) offer complex design and development challenges. A recent area of focus for Abzena to further improve the efficacy of ADCs, and address drug resistance issues, has been the development of dual-payload ADCs. Our streamlined solutions simplify the development process. Interconnected teams collaborate, offering full end-to-end support – all under one organization, accelerating your timelines to clinic and commercial manufacturing, specializing in:
We ensure a robust and consistent bioconjugate manufacturing process from clinical to commercial scale. Our capabilities include:
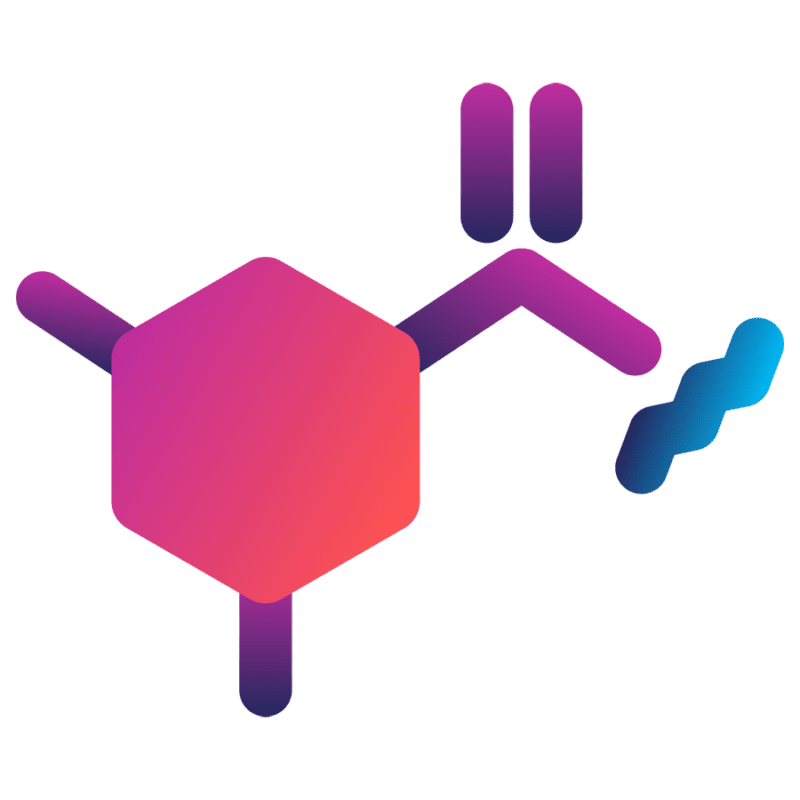
Linker technologies are critical when developing of Antibody-Drug Conjugates (ADCs). Most linkers, particularly those used in first-generation ADC bioconjugation have limitations. The impact, safety and efficacy of the ADC can be compromised. ThioBridge™ was developed by Abzena to overcome those limitations and improve the design and delivery of ADCs.
Building on the unmatched expertise of our scientists, the IP we’ve generated, and our advanced facilities in the UK and US, we have everything you need to implement fully integrated commercial bioconjugation & ADC development and manufacturing programs.
We offer access to genuine scientific experts, streamlined development solutions, interconnected teams and full end-to-end support – all under one organization.

Bioconjugation has emerged as a cornerstone technology, enabling precise drug delivery, whilst enhancing therapeutic efficacy and reducing adverse effects. In our latest whitepaper our bioconjugation experts have explored the variety of conjugate types that are now accessible, each designed to enhance medical outcomes in various therapeutic areas.
Moving medicines forward created with bioconjugates such as ADCs, AOCS and RDCs, and linker payloads is where we excel. Abzena is the leading, fully integrated, bioconjugate CDMO + CRO services provider through to commercialization.
Bioconjugates are hybrid molecules linking biological and chemical components. They include antibody-drug conjugates (ADCs) and antibody-oligonucleotide conjugates that can be used in highly targeted therapies.
Bioconjugation or bioconjugate chemistry as it can also be referred to, is the chemical process of precisely linking two (or more) molecules, where at least one component is a biologically active molecule with a potent payload. The goal of bioconjugation is to create a new and stable targeted therapy, a ‘bioconjugate’ such as an ADC or AOC.
Yes, Abzena has experience through multiple AOC programs, with expertise in antibody formats (mAbs, Fabs, VHHs), analytics and biological assessment such as cell binding, trafficking and gene knock-down.
ADCs and AOCs share structural similarities. They both combine antibodies with functional payloads. Their development processes differ significantly due to the complexity of oligo payloads in AOCs. These differences present distinct technical, analytical and regulatory challenges that experienced developers like Abzena must address.
The process starts by defining the molecule’s function and development stage, then designing tailored conjugate panels to guide discovery and optimization.
For AOCs, regulatory frameworks are continuing to evolve. Developing AOCs involves navigating a regulatory landscape that is incomplete. The FDA and EMA classify oligos differently. The unique characteristics of AOCs – particularly their oligo payloads – present challenges that existing frameworks do not fully address. This gap requires experienced bioconjugate developers such as Abzena to adopt proactive and scientifically rigorous approaches.
Yes, if a suitable handle is available for linker attachment, Abzena can conjugate your payload to a monoclonal antibody and guide you through the process.
Yes, Abzena offers end-to-end tech-transfer support for ADC, AOC, and other conjugation programs, helping companies onshore their processes while maintaining consistency, quality, and scalability.
The leading biologics CDMO + CRO that accelerates drug development, moving medicine forward for patients in need.