Why Abzena?
Trust our focused approach.

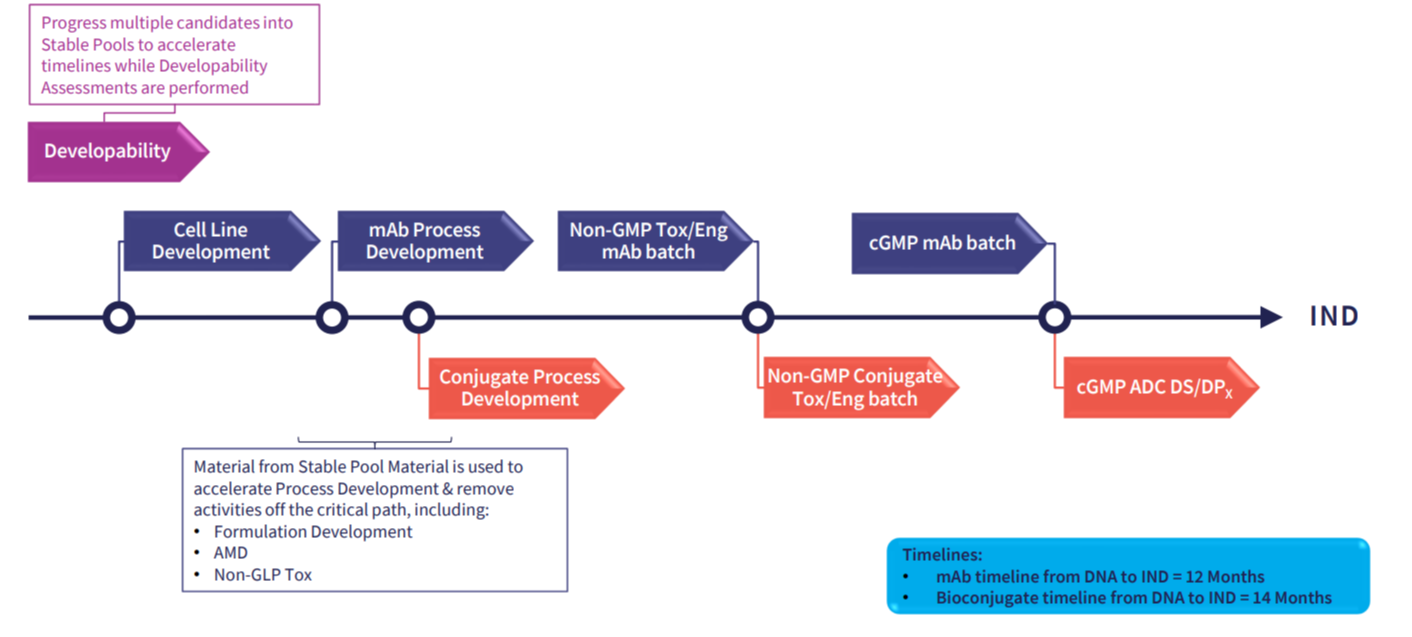
Move from DNA to RCB in 10 weeks. Our CLD offering is fully portable, has no Abzena exit fees, no royalties and a single milestone payment at IND*. Discover our enhanced, mammalian cell line development platforms - AbZelectPRO™, AbZelectPRO™-KO, & AbZelectPRO™-KO+.

High-quality mAb titers up to 10g/L
>90 INDs & 4 marketed drugs with Revvity GS KO & >20 INDs with 2G UNic® Vector

Go from DNA to RCB in 10 weeks
No Royalty Fees & No Exit Fees
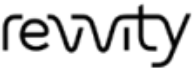

* A Strategic Partner Fee is applicable if/when license holder transfers asset to another party.
Why afucosylated antibodies?
Elimination of the fucose in the Fc glycan structure is known to enhance Antibody Dependent Cellular Cytotoxicity (ADCC) activity of NK effector cells by increasing binding to the FcγRIIIa (CD16a) receptor.
With over 20 years of experience and a commitment to quality excellence, our dedicated CLD team has developed hundreds of cell lines that have rapidly progressed from IND through to clinical trials.
Our mammalian cell line development offering includes:

Each CLD project is tailored to your needs and handled by a dedicated team that works with you from project kick-off to completion. Whether you’re in need of fast stable pool material to generate data or a fully integrated CLD program, our team works with you to get an in-depth understanding of your molecule to determine the best strategy to reduce timelines without compromising quality.
Find out how our newly enhanced CLD solution provides the foundation of biopharmaceutical development success by downloading our new info sheet.

Our unique standalone or fully integrated CLD approach encompasses three key steps to ensure a robust and scalable cell line:
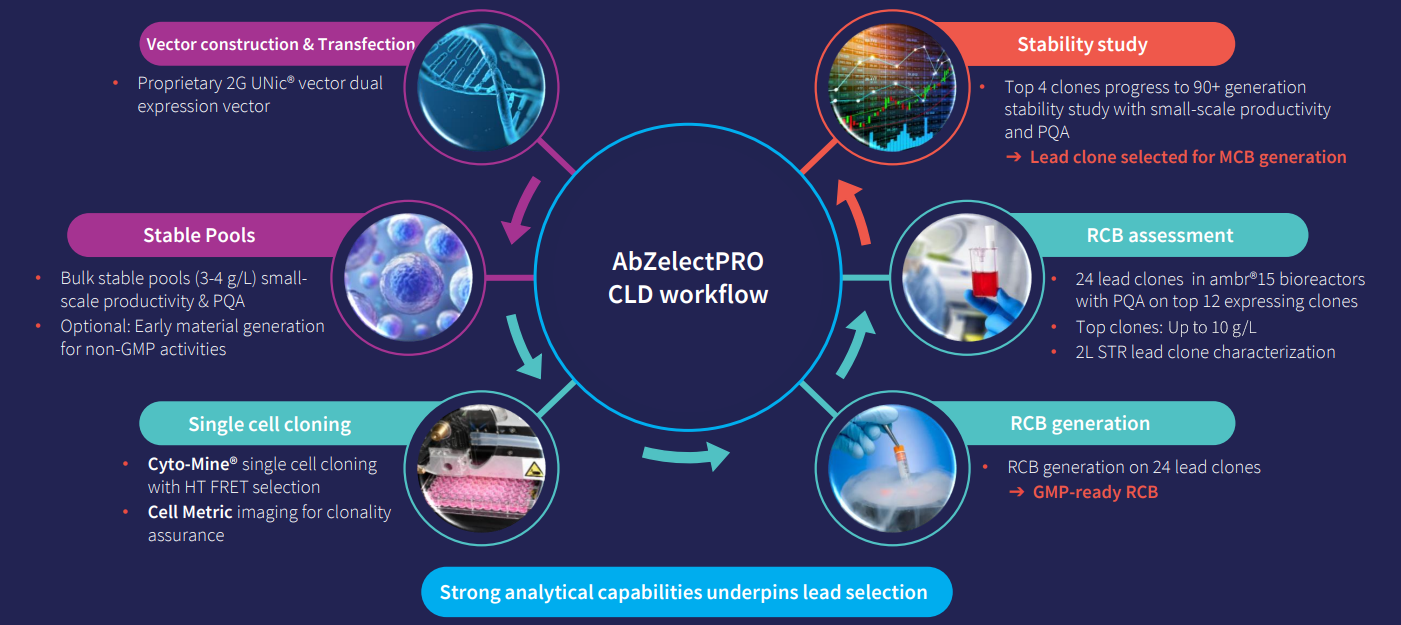
Having high quality material is essential to generate data you can trust in early development. At Abzena, we have a variety of options for generating material that are stage-appropriate from mg to gram scale for fast stable pools to remove activities off the CLD critical path. Our experts in downstream bioprocessing laboratory are well equipped to provide up to 10g of non-GMP shake-flask material that can either support:
Key elements include:
Stage-appropriate, fit-for-purpose assessments & technologies provide in-house analytical solutions.
Abzena offers state-of-the art analytical capabilities to support CLD. We understand that analytical methods are essential to all aspects of pre-clinical drug development, informing critical decisions and de-risking subsequent development phases. For CLD, we use advanced screening assays to identify and select top-performing clones for robust protein expression.
This includes measuring product titers, evaluating growth characteristics, and performing early-stage quality checks – such as glycosylation profiling or charge heterogeneity – to ensure each clone meets specific project goals based on the target product profile (TPP).
Key in-house capabilities include:

Benefits
AbZelectPRO™ is our enhanced CHO-K1 cell line development platform, offering high yields and rapid progression from DNA to RCB in 10 weeks. Our GS Knockout (KO and KO+) technologies further enhance stability and efficiency, de-risking the IND journey.
Our AbZelectPRO™ platform supports efficient, stable production of both traditional and difficult-to-express therapies such as fusion proteins, bispecifics, and other novel modalities by combining ProteoNic’s 2G UNic® premium vector technology with a CHO host cell line. Further enhanced with our GS Knockout (KO and KO+) systems, it features a unique double promoter to drive gene expression and mRNA stability, achieving higher viable cell density and productivity than other platforms.
Our AbZelectPRO™ platforms are designed to provide the flexibility needed to support your unique antibody-based therapy, whether that’s a monoclonal antibody (mAb), bispecific antibody, fusion protein or a novel modality. Leveraging our analytical development and regulatory expertise, we can adapt our testing methods or develop new assays to safeguard the quality of your therapy from CLD to manufacturing.
For standard IgG mAbs, Abzena can reach titres up to 10 g/L at the ambr15 clonal assessment stage, with research cell banks ready in as little as 10 weeks from transfection.
Abzena uses a random integration approach, which delivers stable, high-yielding clonal lines comparable to transposase methods. Our capability is further enhanced with our two new GS Knockout cell lines. We guide clients toward context-driven CLD decisions. Our findings empower drug developers to reconsider assumptions and embrace the method best suited for their molecule, timeline, and budget. Please download our detailed whitepaper including recent case studies following the link below.
Abzena’s CLD platform has advanced more than 20 programs into the clinic. With proven performance, more than 90 INDs and four marketed drugs.
Efficient tech transfer is a common area of focus when relying on a contract development and manufacturing organization (CDMO) for support, being critical for the smooth transition of projects from CLD into manufacturing. Operating across our sites in Cambridge, UK, and San Diego, US, our teams work closely together to ensure the seamless progression of your project. Our extensive experience and collaborative culture allow us to proactively identify and prevent risks that could delay your therapy reaching patients.