Why Abzena?
Trust our focused approach.

We leverage advanced chemistry & complex biologics expertise to design, develop, and manufacture Radioconjugates including Antibody-Chelate Conjugates (ACCs) that can be used to load radioactive elements for use as cancer therapies or in imaging studies such as PET scanning.
As the industry increasingly focuses on targeted radiotherapies, our integrated approach ensures that your program is built on a robust foundation while being optimized for safety, scalability, and regulatory compliance.

Radionuclide Drug Conjugates (RDCs) combine three key elements:
Our comprehensive service offering covers every stage of Antibody chelator conjugate development and manufacture:
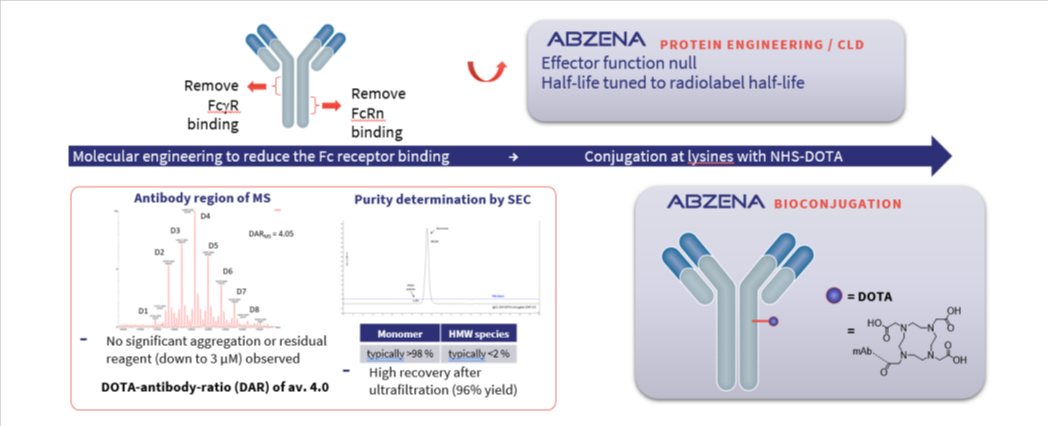
Our approach is defined by four key differentiators:

Contact us to learn how Abzena’s integrated solutions and deep technical expertise can transform your targeted radioconjugate development.