Why Abzena?
Trust our focused approach.
Our expertise spans every component of modern bioconjugate design, including antibodies and targeting proteins, conjugation chemistries, polymers, release mechanisms, and payloads. We provide full discovery and ADC design support, including antibody-drug conjugates (ADCs), antibody-oligonucleotide conjugates (AOCs), radionuclide-drug conjugates (RDCs) including antibody-chelate conjugates (ACCs) and more.
Abzena’s preclinical discovery scientists have deep experience in developing robust, reproducible conjugation methodologies. We work collaboratively to optimize your molecule’s stability, efficacy, and safety profile from early research through preclinical testing.

Abzena & Mabqi have formed a strategic partnership that will integrate Mabqi’s antibody discovery capabilities with Abzena’s bioconjugate development and manufacturing services, offering our customers a more streamlined, end-to-end drug development solution.
The partnership will leverage Mabqi’s LiteMab Antibody Discovery Studio for hit screening, characterization, and hit selection by using universal & pH-sensitive libraries to identify top lead candidates, and utilize Abzena’s bioconjugate capabilities for developability, cell line development, process development, and GMP manufacturing.
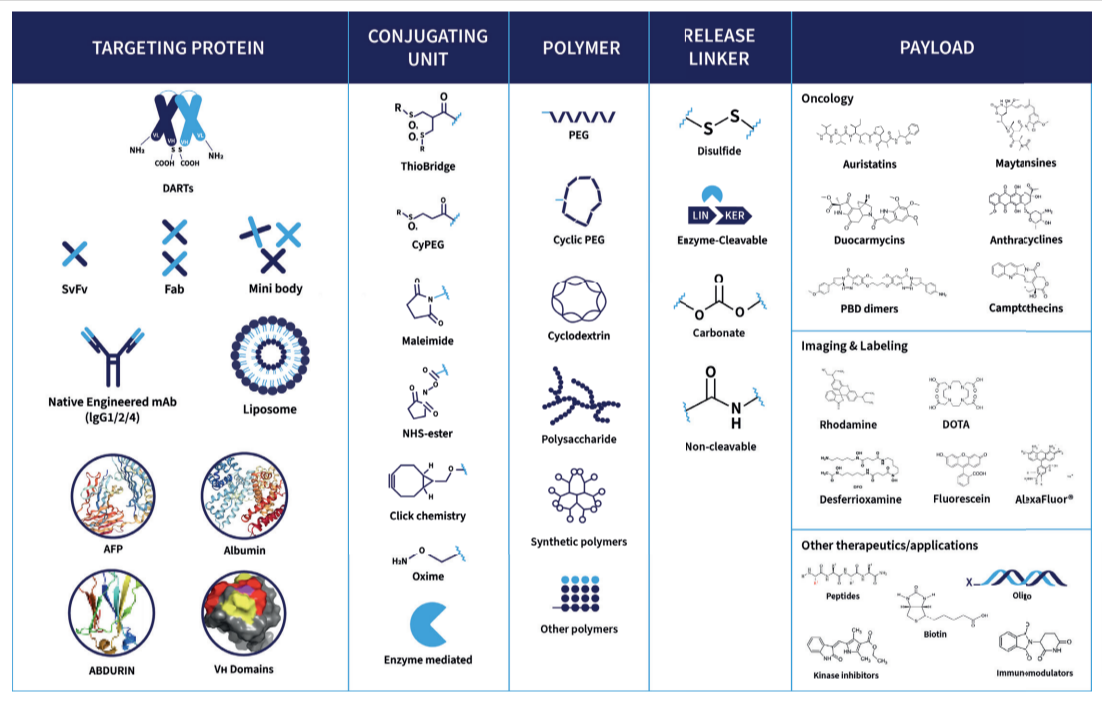

To optimize each molecule, Abzena’s expert scientists apply extensive experience in bioconjugates design to deliver the desired mode of action and achieve the defined Target Product Profile (TPP) for your program.
Our teams work with a wide range of target carriers, including full antibodies, antibody fragments, engineered scaffolds, peptides, and other carrier proteins. This flexibility supports the design of complex conjugates such as antibody-drug conjugates (ADCs), antibody-oligonucleotide conjugates (AOCs), and radionuclide-based constructs.
We take a holistic approach to ADC design, considering all variables from carrier selection to ADC linker design and payload release. Our deep understanding of protein engineering allows us to enhance targeting precision, stability, and functionality – ensuring each molecular entity performs as intended in preclinical development and beyond.
To deliver the required biological activity for your program, with efficacy and safety in balance, Abzena’s scientists apply deep experience in bioconjugates design and payload selection.
Our teams have worked with a wide range of cytotoxic payloads, including auristatins, maytansines, anthracyclines, amatoxins, PBD dimers, duocarmycins, and camptothecins. We also support payloads used in emerging therapeutic modalities such as immunomodulators (TLR and STING agonists), peptides, polysaccharides, antibody-oligonucleotide conjugates, DNA and RNA-based oligomers, and chelators for radionuclide-drug conjugates and targeted radioimmunotherapy.
Through precise ADC linker design, we mitigate challenges caused by variations in payload chemistry and stability, ensuring consistent conjugation and controlled release.
A comprehensive suite of analytical methods supports each stage of design and development, enabling accurate characterization and performance evaluation of your conjugate.
We provide a range of both proprietary and non-proprietary PEGylation technologies for use with different molecular weight PEGs, in linear and branched formats.
They have been designed to conjugate PEG to either disulfide bonds, thiols on single cysteines or to poly-histidine motifs.
We provide a range of both proprietary and non-proprietary PEGylation technologies for use with different molecular weight PEGs, in linear and branched formats.
They have been designed to conjugate PEG to either disulfide bonds, thiols on single cysteines or to poly-histidine motifs.

Abzena’s expert team applies advanced bioconjugates design principles to develop a wide range of tailored conjugates. We routinely prepare bioconjugates using traditional chemistries that stochastically target lysine or cysteine residues, enabling reproducible and scalable ADC design strategies.
In addition, our scientists have deep experience with site-specific conjugation approaches, including:
For proof-of-concept evaluation, linker assembly utilizing click chemistry allows for rapid and efficient modulation of its constitutive elements to evaluate:
Our experts develop robust conjugation processes maximizing conversion to the target species and purification strategies allowing isolation with high recoveries and purity.
Critical Process Parameters (CPP) are identified at an early stage to ensure development of a conjugation process, accelerate your lead candidate, and scale up of your manufacturing.
Abzena are leaders in providing specialized bioconjugation services across ADCs, AOCs, RDCs, ACCs and more, all underpinned by robust analytical capabilities.
Bioconjugates are the process of linking biologically active molecules to payloads for targeted delivery. Abzena designs stable, effective conjugates with precise control over release and activity.
Our teams apply integrated ADC design and ADC linker design principles to balance potency, stability, and safety, optimizing linkers for controlled payload release.
We support bioconjugates design for cytotoxins, immunomodulators, and oligonucleotides, as well as radionuclide drug conjugate development for radioimmunotherapy.
Using validated conjugation and analytical methods, Abzena ensures each bioconjugate is consistent, pure, and ready for regulated development.

The asset you’re developing right now can make a real difference for human health. Let us use our wealth of experience to deploy the best technologies, shorten lead times and implement effective risk mitigation strategies.