Why Abzena?
Trust our focused approach.

Rapidly progress your large molecule from DNA to RCB in 10 weeks. Fully portable CLD, no Abzena exit fees, no royalties, & a single milestone payment at IND*. Now available with GS Knockout AbZelectPRO™-KO, and AbZelectPRO™-KO+ with ADCC+.



There are three key aspects of CLD which have been optimized in our AbZelectPRO™ platform:
* A Strategic Partner Fee is applicable if/when license holder transfers asset to another party.

For more complex biologic and bioconjugate programs, we can deliver increased productivity with a >30% reduction in cell line development timelines using our AbZelectPRO™ platform. AbZelectPRO™ combines our existing CHO cell line with ProteoNic’s premium expression vector technology 2G UNic.
Download our Info Sheet now to discover how our AbZelectPRO™ platform streamlines cell line development, enhancing efficiency and cutting your timelines to IND application. Tailored for small/mid biotechs with antibody-based therapeutics.

We have an integrated cell line development approach which shortens timelines to IND enabling material. This is supported by using fast stable pools to generate early-stage material for characterization, pre-formulation, analytics and lead molecule selection.
Technical Evaluation of AbZelectPRO™
Benefits of AbZelectPRO™ with 2G UNic vector technology:
ProteoNic’s 2G UNic technology uses the combined effect of novel genetic elements to exert a positive effect on recombinant protein production levels and boosts the performance of other expression-enhancing technologies. This state-of-the art vector technology also increases production levels of difficult-to-produce complex protein, including bispecifics and fusion proteins as well as levels of products already in the multiple g/L range.
To achieve the highest production levels, AbZelectPRO™ combines our CHO-K1 mammalian cell line with ProteoNic’s 2G UNic® premium vector technology to boost expression levels and generate higher-producing, stable cell lines up to 10g/L of product.
As a result, the AbZelectPRO™ platform supports the efficient and stable production of antibodies and more difficult-to-express proteins such as fusion proteins, bispecifics and other novel modalities.
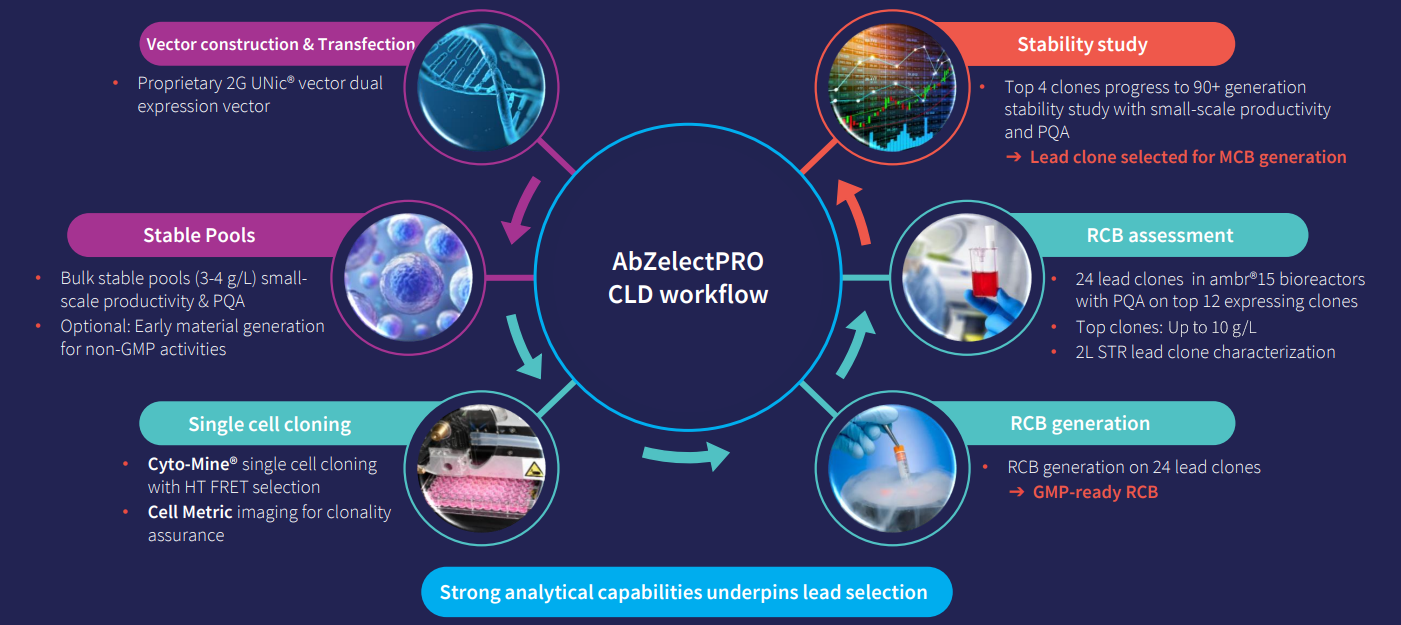

Designed for both traditional and difficult-to-express protein programs, such as antibodies, bispecific, and fusion proteins, Abzena’s AbZelectPRO™, is optimized across the three key areas of vector, host cell line, and process. It provides improved cell doubling times, viable cell densities, and robust and stable cell lines.

Our AbZelectPRO™ platform combines AbZelect™ with 2G UNic® premium vector technology to boost expression levels and generate higher-producing, stable cell lines expressing proteins including bispecifics, fusions and other novel modalities.

By streamlining the CLD process, our AbZelectPRO™ platform can progress your project from sequencing to research cell banks in 10 weeks with 10g/L in titres, helping to deliver your project to the clinic faster.

AbZelectPRO™ promotes the in-depth characterization of multiple candidates at early stages through the generation of multiple fast stable pools, enabling rapid identification and mitigation of risk.
Our AbZelectPRO™ platform supports the efficient and stable production of both traditional and difficult-to-express therapies like fusion proteins, bispecifics and other novel modalities by combining ProteoNic’s 2G UNic® premium vector technology with host CHO cell line. Offering a unique double promoter to drive gene expression and enhance messenger RNA stability, the AbZelectPRO™ platform achieves higher viable cell density (VCD) and productivity in comparison to other cell line platforms.
Efficient tech transfer is a common area of focus when relying on a contract development and manufacturing organization (cdmo) for support, being critical for the smooth transition of projects from CLD into manufacturing. Operating across our sites in Cambridge, UK, and San Diego, US, our teams work closely together to ensure the seamless progression of your project. Our extensive experience and collaborative culture allow us to proactively identify and prevent risks that could delay your therapy reaching patients.
By integrating fully automated, next-generation technologies such as the Cyto-Mine® high-throughput microfluidics screening platform into AbZelectPRO™, we can rapidly select high-performing clones while ensuring monoclonality. The Cyto-Mine® platform provides productivity data on a per-cell basis, allowing us to rank individual clones and select single cells with the highest productivity for further expansion and characterization.
The AbZelectPRO™ platform is designed to provide the flexibility needed to support your unique antibody-based therapy, whether that’s a monoclonal antibody (mAb), bispecific antibody, fusion protein or a novel modality. Leveraging our analytical development and regulatory expertise, we can adapt our testing methods or develop new assays to safeguard the quality of your therapy from CLD to manufacturing.