Why Abzena?
Our focused approach.
Author: Dr Petra Dieterich, SVP & Scientific Leader
In a recent article on BioProcess Online, our SVP and Scientific Leader, Dr Petra Dieterich, provides details around our unique, site-specific conjugation technology, ThioBridge™, and how it can improve both the design and delivery of ADCs, AOCs, and RDCs.
The drug conjugate landscape is broadening its horizon with variations on traditional Antibody-Drug Conjugates (ADCs), including bispecific ADCs, dual payload ADCs, antibody-oligo conjugates (AOCs) and radionuclide conjugates (RDCs) amongst the modalities in clinical development as novel oncology treatments.
While most commercially approved ADCs have relied on stochastic lysine and cysteine conjugation technologies, the trend is for the use of conjugation technologies that provide conjugates with linkers that retain payload stability with homogeneous Drug-to-Antibody loading (DAR). Technologies that make use of the naked antibody are also favored because they avoid antibody engineering and can tap into existing supply chains. Technologies that offer versatility with both DAR and payload can also be favored in the discovery process because they lend themselves to structure activity relationship optimization.
Only a few methods exist whereby the payload is loaded homogeneously onto the native antibody, and this is the cysteine re-bridging approach at the native interchain cysteines and results in conjugates with highly homogeneous DAR. The technology uses a two-step sequence. Firstly, the reduction of the interchain disulfides to release the sulfhydryl groups for nucleophilic attack. In a typical IgG1, the 4 reduced disulfides will provide 8 free sulfhydryl units. The second step is reaction with a bis-functional electrophile capable of reacting with the sulfhydryl residues to covalently re-bridge the disulfide bonds. Once fully re-bridged, the resulting conjugate will bear exactly one conjugated linker per disulphide bond and with one payload per reagent molecule, this will generate ADC’s with very clean drug-to-antibody ratio as shown in the HIC profile in the Scheme. Abzena’s ThioBridge™ site-specific conjugation technology is one of these such technologies.
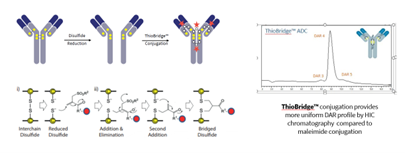
Scheme: ThioBridge conjugation is through the interchain disuphides on the native antibody and provides clean DAR by HIC chromatography
The stability of ThioBridge™ conjugated ADCs vs maleimide conjugated ADCs has been demonstrated in serum ex vivo. As shown in the scheme, analytical size-exclusion chromatography (SEC) analysis revealed that the ThioBridge™ conjugate maintained its stability, with no loss of the linked AlexaFluor 488 while the maleimide conjugate showed payload loss.
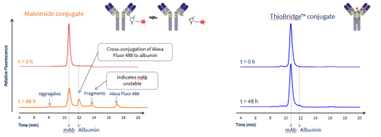
Scheme: Size-exclusion chromatography (SEC) analysis demonstrating the stability of ThioBridge™ vs Maleimide conjugation
Comparing the potency of the ADC HTI-1511, generated via ThioBridge™ conjugation with a maleimide ADC in tumor mouse models (Scheme X) shows that ThioBridge™ generated ADCs exhibit higher potency.
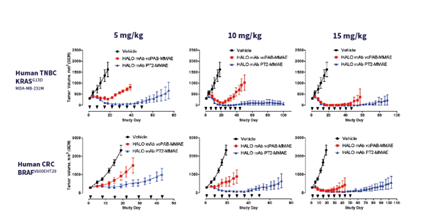
Scheme: Comparing HALO mAb vCPAB MMAE (maleimide linked) with HALO mAb PT2 MMAE (ThioBridge™ linked, HTI-1511)
The ThioBridge™ reagent architecture includes branching points which allows the loading of two, or more, payload molecules per reagent. Alongside this, side chain units such as PEG, cyclodextrins and cyclic PEB side chains can be incorporated, as shown in the scheme below.
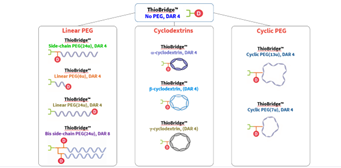
Scheme: Examples of different ADC formats and side chains that can be generated through ThioBridge™ conjugation
In a comparative study, a DAR 8, bis PEG, ThioBridge™ conjugated ADC was compared with a maleimide ADC containing a glucuronide release linker. As shown in the figure below. Both ADCs gave complete responses at the 1mg/kg dose. However, at the 0.5mg/kg dose, the ThioBridge™ ADC gave superior efficacy.
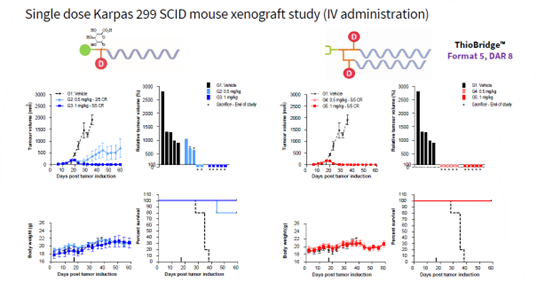
Scheme: Comparison of ThioBridge DAR 8 bis PEB ADC with Maleide glucuronide ADC
In summary, ThioBridge™ site-specific conjugation technology has the versatility to support a number of different conjugation modalities including bispecific ADCs, dual-payload ADCs, AOCs and RDCs. Versatility in the reagent architecture allows for variation around DAR, payload and side chain. To date, seven ADCs containing ThioBridge™ conjugation have obtained regulatory approval for use in clinical studies. Scale-up of the chemical processes involves standard and tested operations that do not require enzymes and are optimized to avoid chromatography.
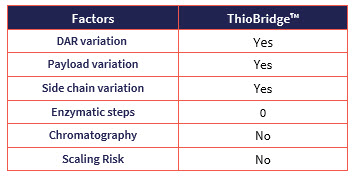
Table: Key properties of ThioBridge™ linker technology
