Leadership Team
Meet our experienced team.
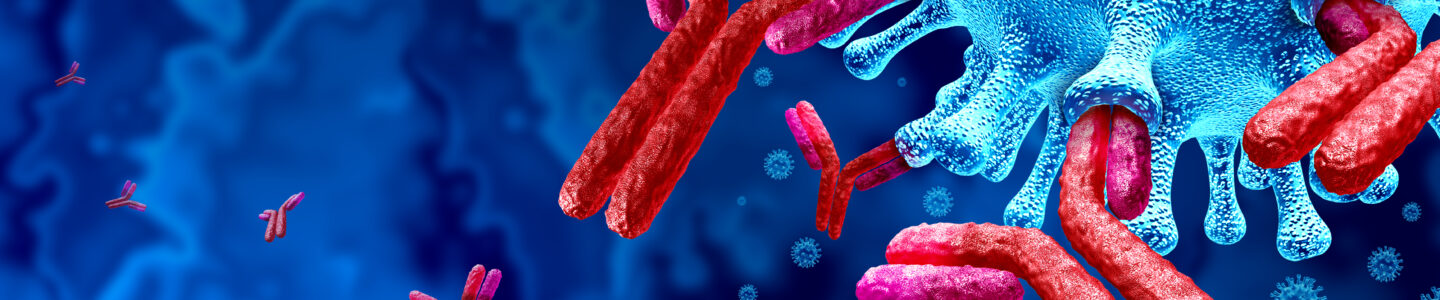
Antibody-drug conjugates (ADCs) are proving to be a game-changer in the world of cancer treatment, offering targeted delivery of potent drugs directly to cancer cells, while sparing the healthy ones.
Dual-payload ADCs are beginning to push the boundaries of what ADCs can really achieve in the clinic. They carry not just one, but two types of therapeutic agents, aimed at overcoming diverse challenges like tumor heterogeneity and drug resistance. Here we take a journey through the evolution of ADCs, focusing on dual-payload innovations that are setting the stage for the next generation of cancer therapy.
The Ever-Evolving Payloads
ADCs have come a long way. The first-generation ADCs were paired with traditional chemotherapy agents like methotrexate and doxorubicin1. While good in theory, these combinations had insufficient toxicity and didn’t live up to their potential. Then came the second-generation ADCs, which used advanced payloads like tubulin polymerization inhibitors 2 and also maytansinoids and auristatins3. Although highly effective against certain types of fast-growing cancer, these too had their limitations, especially against slower-growing cancers.
To tackle this, researchers started experimenting with DNA-damaging agents like pyrrolobenzodiazepine (PBD) dimers4 and topoisomerase inhibitors5. Yet the trade-off between potency and side effects remained an issue. The scientific community, however, isn’t one to sit still, and now we’re seeing intriguing developments, like proteolysis targeting chimeric molecules (PROTACs)6 and, most excitingly, dual-drug ADCs7.
A Leap into Novel Mechanisms
As our understanding of tumor biology deepens, the ADC field is venturing into uncharted territory with innovative payloads that target novel mechanisms of action. HSP90 inhibitors, for instance, are making a comeback with refined structures that show promising results in early trials8,9. These inhibitors join other exciting advances like translation10 and proteasome inhibitors11, each presenting their unique challenges and benefits but all aimed at overcoming the limitations of traditional cytotoxic agents.
Perhaps most intriguingly, the introduction of Proteolysis Targeting Chimeras (PROTACs) and immune modulators as ADC payloads marks a paradigm shift. These not only inhibit but actively dismantle cancer cell machinery or recruit the body’s own defenses for a multi-faceted attack. While challenges remain in fine-tuning their specificity and potency, these novel mechanisms open new avenues for targeted cancer therapy, driving us closer to a future where ADCs can adapt to the complex and diverse landscape of oncological diseases12.
The Era of Dual-Drug ADCs
But here’s where it gets really interesting. Enter dual-drug ADCs. This approach is groundbreaking because it recognizes that cancer is a complex beast. From tumor heterogeneity, or the idea that different areas of a tumor can have distinct characteristics to drug resistance which can often turn an initially effective treatment ineffective, the hurdles are significant.
The idea behind dual-drug ADCs is a bit like a Swiss Army knife of cancer treatment; it’s designed to deal with multiple issues at once. Some pioneering research has already been done in this area13. For instance, a study developed a dual-drug ADC that contained both monomethyl auristatin E (MMAE) and monomethyl auristatin F (MMAF) connected to a single linker. The result? A more effective treatment against resistant tumor models.
It’s also not just about combining two drugs of the same class. Research is also leaning into mixing payloads with entirely different mechanisms of action. Imagine combining a potent drug with an immune system-boosting agent. You’re not only attacking the cancer cells but also mobilizing your own immune system to join the fight—a two-pronged approach for a multi-faceted enemy14,15.
The Road Ahead: Innovation and Challenges
The future is indeed promising for ADCs, with continued innovations in dual-payload systems and the incorporation of immunomodulatory agents16. As with all scientific endeavours, challenges persist. The delicate balance between potency and side effects is the eternal dance of drug development. But as new avenues like HSP90 and proteasome inhibitors become the focus of ongoing research, the next evolutionary step in ADCs is already on the horizon.
So, what’s next? As researchers and clinicians, we need to continue collaborating, pushing the boundaries of what’s possible, and translating these scientific advances into tangible benefits for patients. With dual-payload systems, immune modulators, and novel targets, ADCs are poised to be at the forefront of a new age of cancer therapy.
To learn more about dual-payload ADCs, click here to read a recently published article in Drug Target Review magazine, by-lined by Dr. Nicolas Camper, Dr. Campbell Bunce and Dr. Johanna Midelet, Abzena.
References
You may also be interested in